Pomalidomide Accord Packaging
- PVC/PVDC/aluminium blister
- Perforated unit dose blisters of 21 x 1 capsules
This website is for UK Healthcare Professionals.
Pomalidomide Accord Product Information
Pomalidomide Accord presentations
Capsule images not to scale
Presentations
Each presentation is available in packs of 21 hard capsules
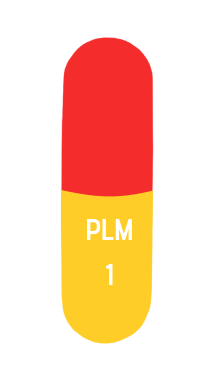
size 4
1mg
14 mm
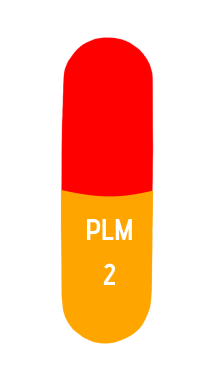
size 2
2mg
18 mm
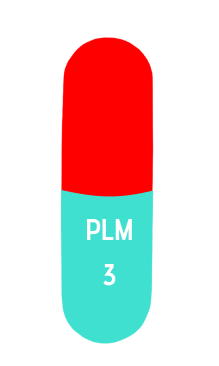
size 2
3mg
18 mm
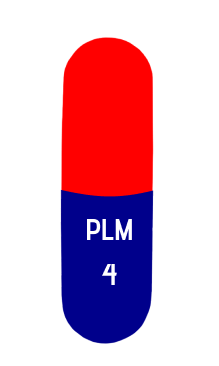
size 2
4mg
18 mm
Shelf life
3 years
Pomalidomide Accord Packaging
Launched November 2024
Pomalidomide Accord hard capsules
Offering the same range of strengths and for the same indications as Imnovid®*5, 6
Differentiated packaging to aid dispensing
Lenalidomide Accord Product Information
Capsule images not to scale
Presentations
Each presentation is available in packs of 21 hard capsules
size 5
2.5mg
11.0 mm to 11.8 mm
size 5
5mg
11.0 mm to 11.8 mm
size 4
7.5mg
14.0 mm to 14.8 mm
size 3
10mg
15.4 mm to 16.2 mm
Shelf life
3 years
size 2
15mg
17.4 mm to 18.2 mm
size 1
20mg
19.0 mm to 19.8 mm
size 0
25mg
21.0 mm to 21.8 mm
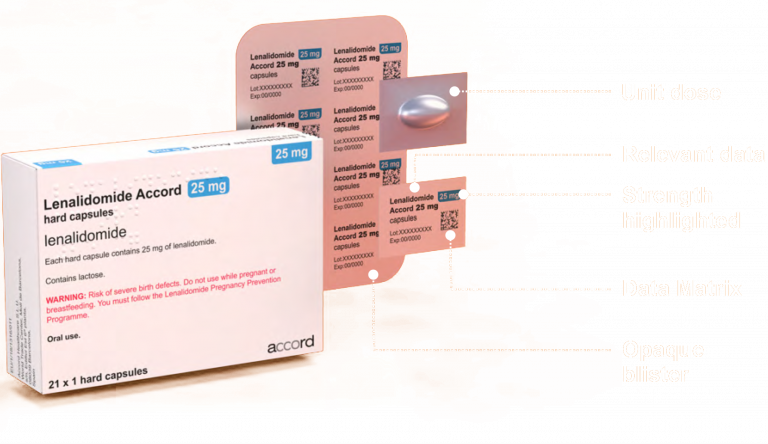
Lenalidomide Accord Packaging
Cross-perforated unit-dose blisters
Colour strength differentiated blisters
Data Matrix included on the blister
Launched January 2022
Lenalidomide Accord hard capsules
Offering the same range of strengths and for the same indications as Revlimid®*:3, 4
Differentiated packaging to aid dispensing


Thalidomide hard capsules
Offering the same strength and indication as thalidomide BMS*1,2
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Accord-UK Ltd on 01271 385257 or email [email protected].
References
For UK Healthcare Professionals
If you are a UK healthcare professional and would like more information on thalidomide, lenalidomide or pomalidomide from Accord, please click here
Report Adverse Events
Adverse events should be reported.
Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in Google Play or Apple App Store.
Adverse events should also be reported to Accord-UK Ltd:
Tel: 01271 385257
Email: [email protected]
For more information on thalidomide, lenalidomide or pomalidomide from Accord, please click below
Site intended for a UK audience only. UK-03777 June 2025